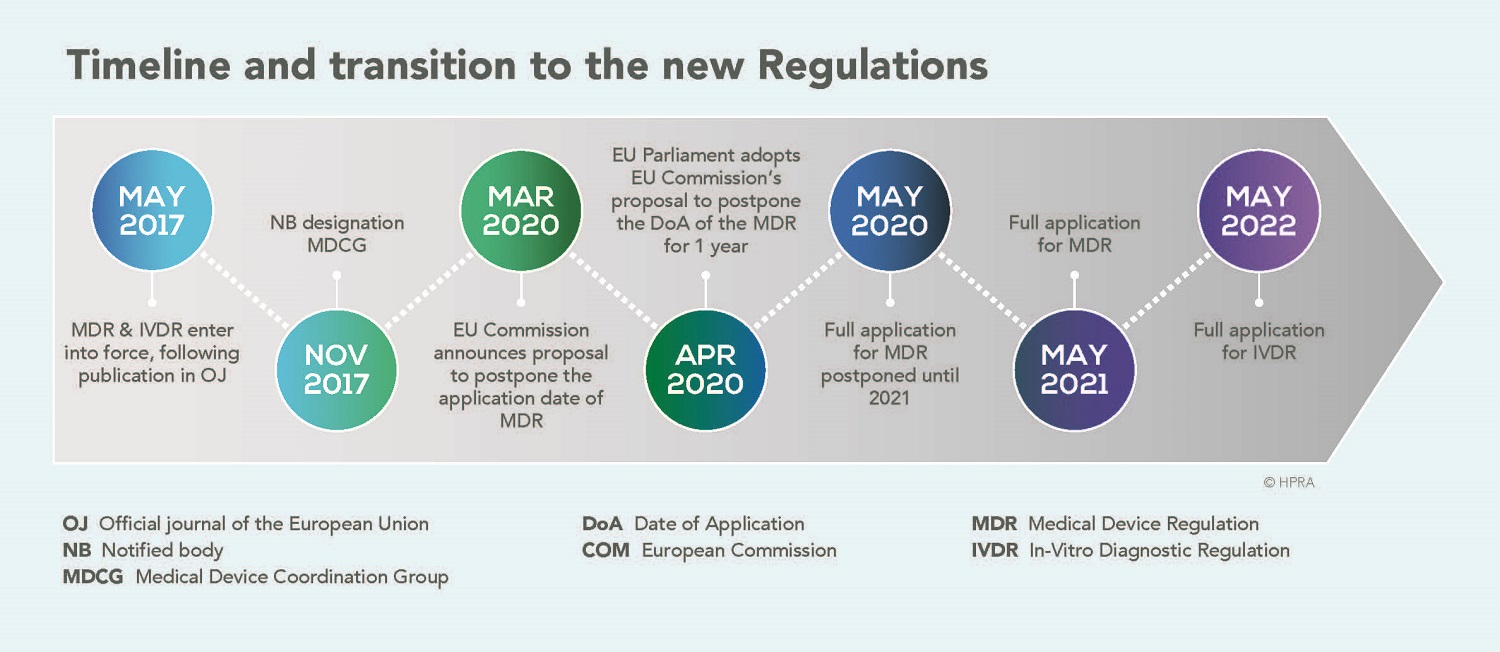
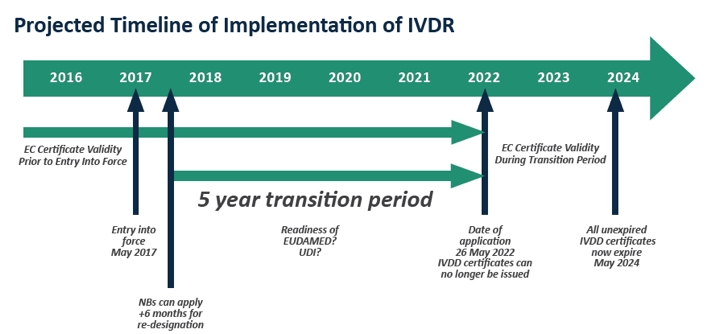
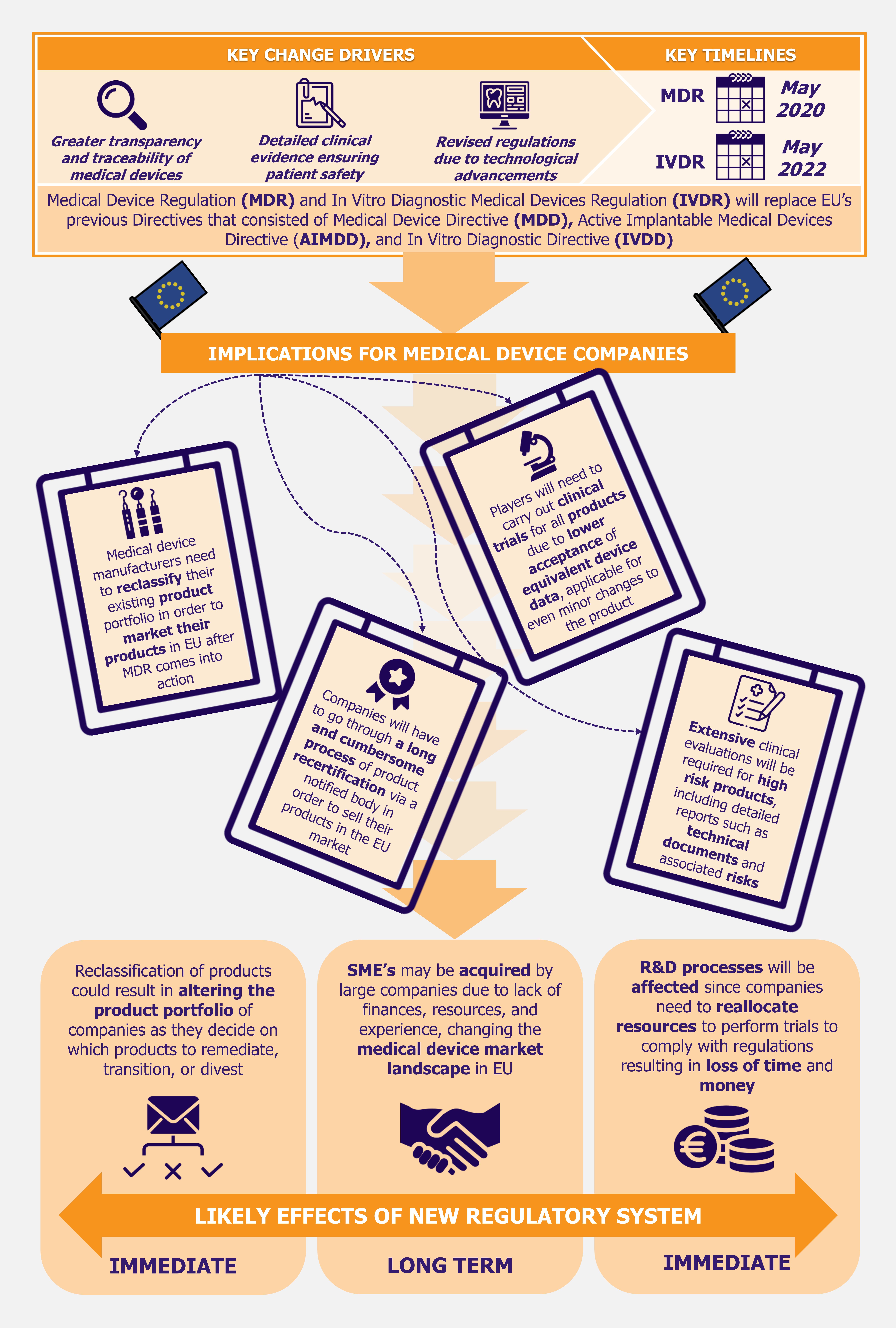
MedTech Europe calls on authorities to ensure a successful transition to the new IVDR - Medical Plastics News

Batman MDR and IVDR update after the first MDCG / stakeholders meeting and an IVDR seminar | medicaldeviceslegal

The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine




/tuv-rheinland-companion-diagnostics-visual-en.png)