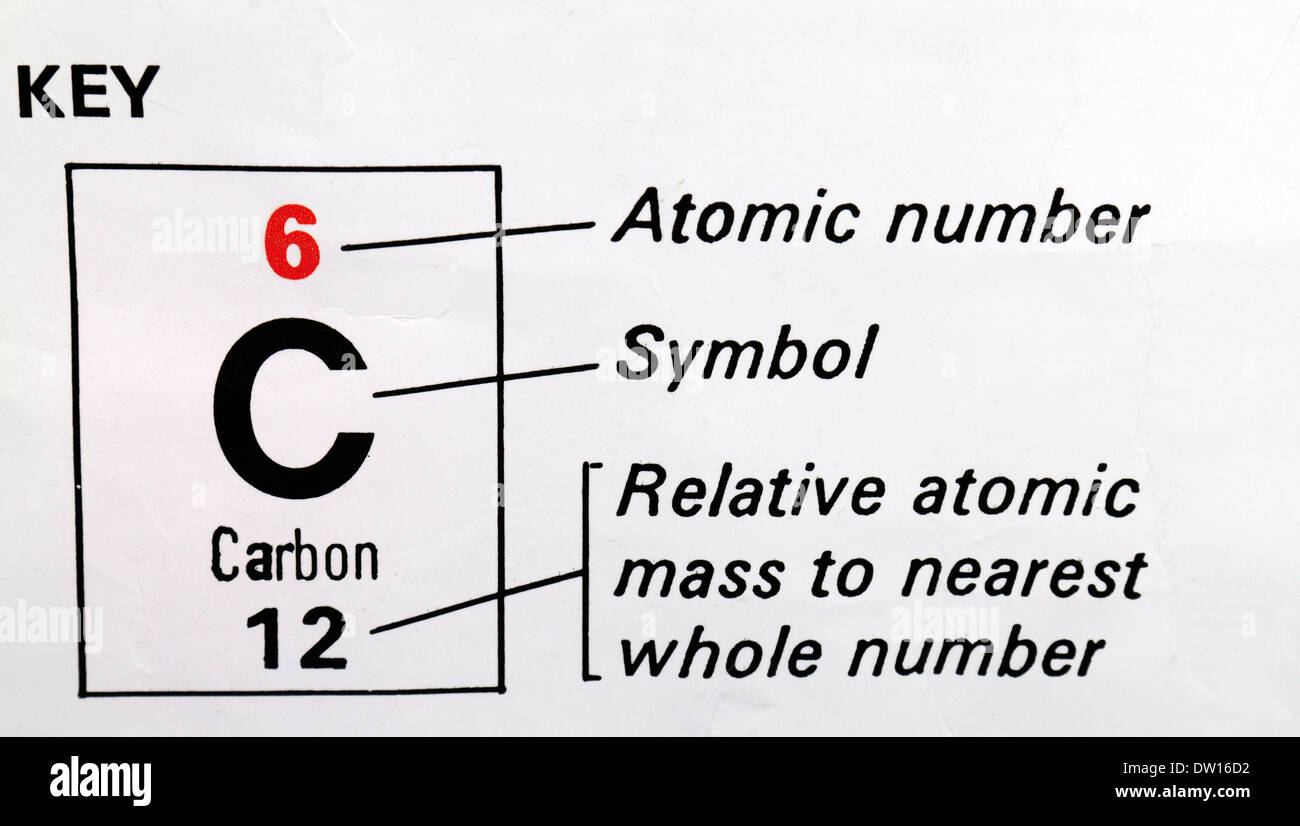
Carbon (C) used as a key on a Periodic Table showing Atomic Number, symbol and Relative Atomic Mass Stock Photo - Alamy

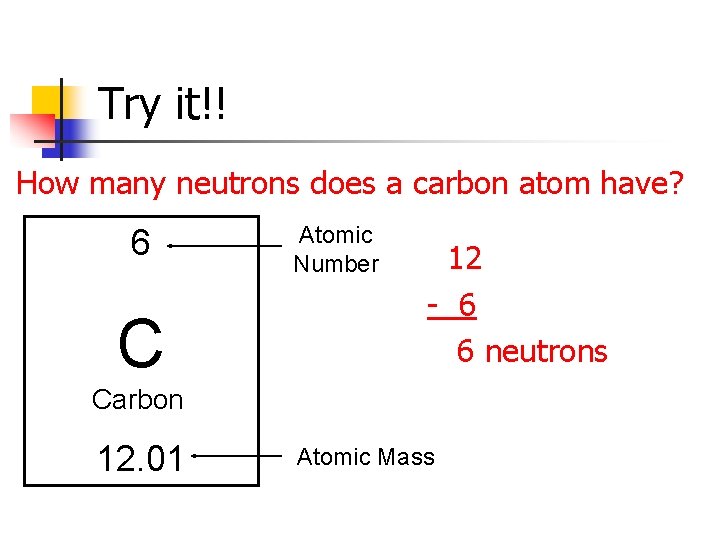
Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download

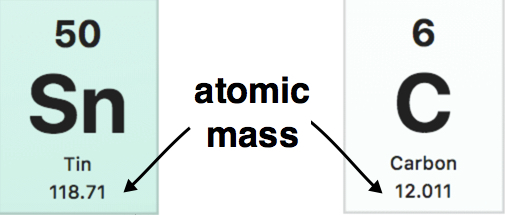



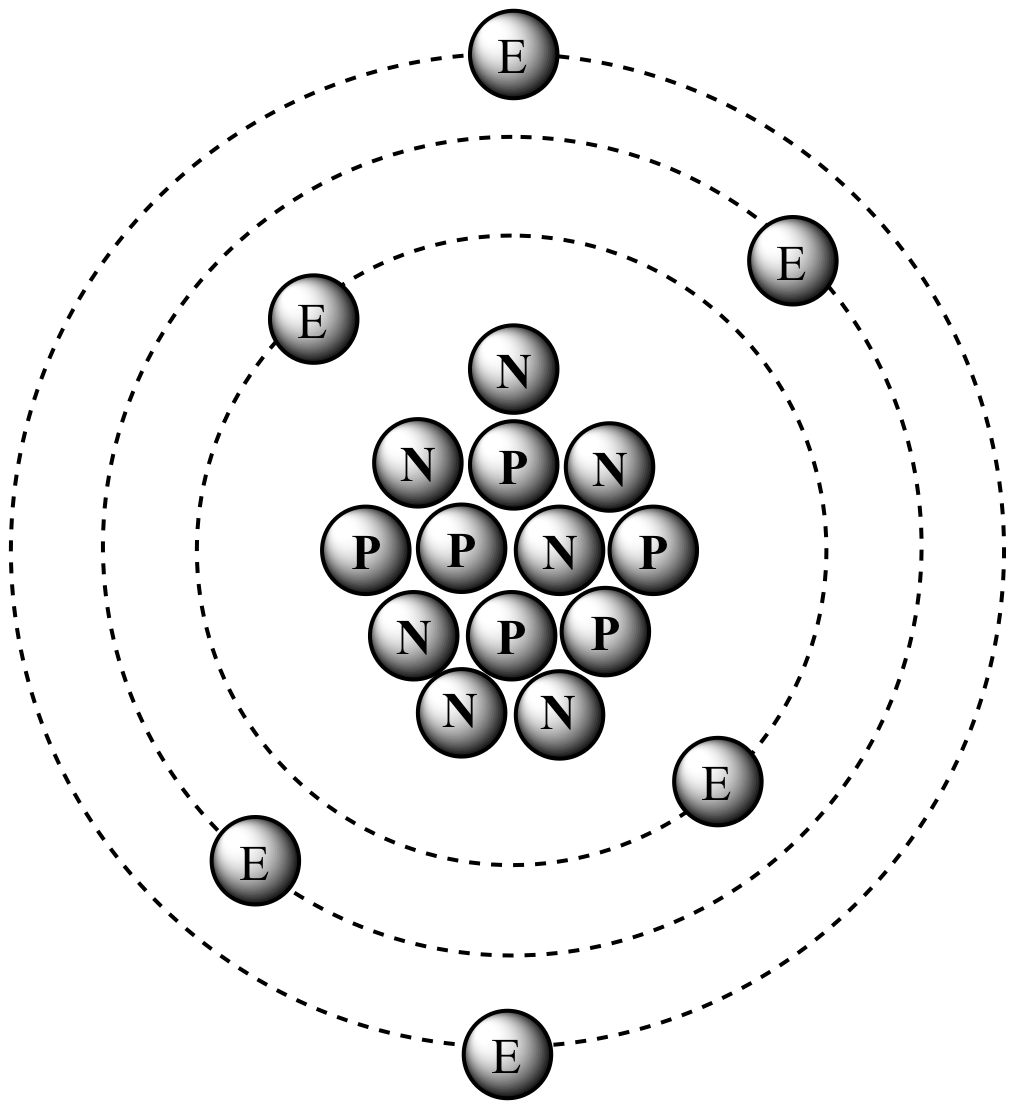
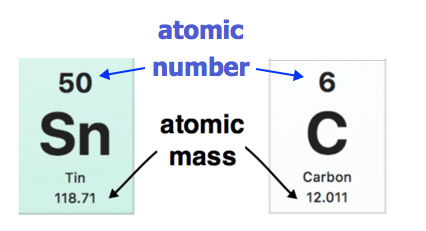
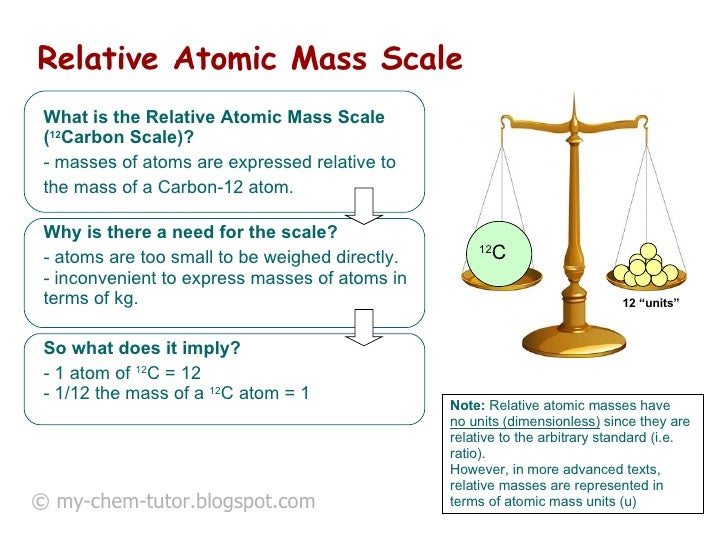
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)